iliff lab research
Sleep, waste and neurodegeneration at the crossroads of the CNS
Everyone knows that they should probably get more sleep. But do the consequences of poor sleep extend beyond feeling tired the next day? Sleep worsens with age, and is frequently disrupted after concussion (mild traumatic brain injury, mTBI). But is worsening sleep part of the reason that the aging and post-traumatic brain are vulnerable to the development of neurodegenerative conditions like Alzheimer’s or Parkinson’s disease, or chronic traumatic encephalopathy? What other cellular changes take place as we age and after mTBI that promote the neuropathological changes underlying these conditions?
These are the types of questions that the Iliff lab seeks to answer. Using cellular and molecular biology, advanced imaging approaches including in vivo 2-photon microscopy, we seek to define the basic mechanisms governing glymphatic exchange and lymphatic clearance of interstitial wastes from the brain, CSF secretion and reabsorption, blood-brain barrier function and cerebral blood flow regulation.
To answer these questions, we make use of rodent models of neurodegenerative diseases and TBI. We also work through diverse network of clinical and bioinformatic collaborators to address these questions using remote sleep assessment, MRI, fluid biomarkers, cognitive assessments in human clinical populations.
The role of post-traumatic sleep-wake disruption in the development of tau pathology following mild traumatic brain injury
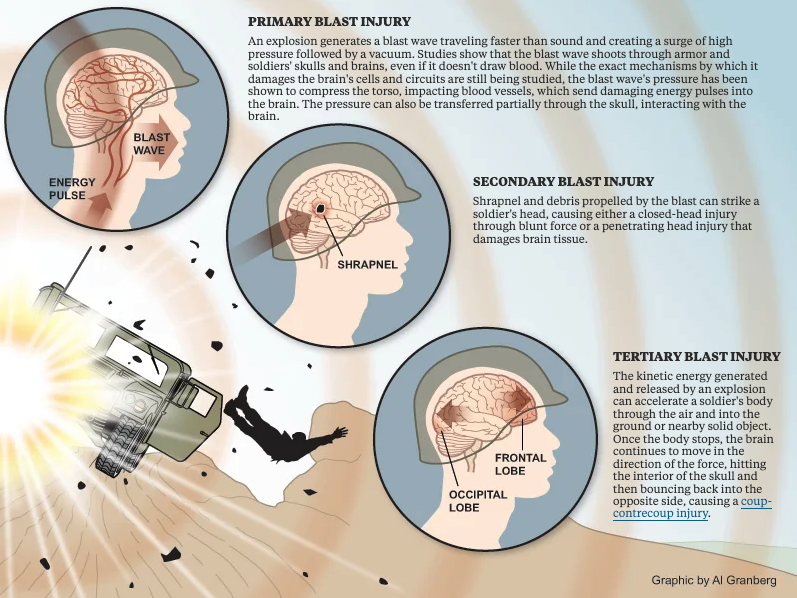
Mild traumatic brain injury (mTBI), or concussion, has emerged as a potential risk factor for the development of neurodegenerative conditions including Alzheimer’s disease. Alzheimer’s disease is characterized in part by the buildup of aggregates of the protein tau within cells of the brain. Yet how mTBI may contribute to the development of this tau buildup remains unknown. Disruption of normal sleep-wake patterns is a frequent complaint after mTBI, while in aging populations, sleep disruption appears to promote the development of neurodegenerative pathology. In this study, we will test whether sleep disruption after mTBI exposure contributes to the development of post-traumatic tau pathology. We will also specifically test whether the dysfunction of the brain’s waste clearance system, called the glymphatic system, links mTBI with tau pathology. These studies may pave the way for new approaches to preventing dementing conditions like Alzheimer’s disease in both Veteran and civilian populations exposed to mTBI earlier in life.
Role of Sleep Disruption after mTBI as a Driver of Chronic Post-traumatic Headache
Disruption of normal sleep and headaches frequently occur after mild traumatic brain injury (concussion) and can persist in some sufferers for months to years following injury. In this proposal, we will be testing whether post traumatic sleep disruption actually contributes to the development of posttraumatic headaches, and whether impairment of the brain’s waste clearing system, the glymphatic system, contributes to these changes. These studies may lead to new approaches to prevent or treat post-traumatic headache by targeting sleep or the function of this cleaning system.
GLIMPSE TBI - Defining the role that sleep disruption and glymphatic impairment play in the development of Alzheimer’s disease pathology after blast mTBI
In a study led by collaborator Dr. Elaine Peskind, this study investigates – in Iraq/Afghanistan combat Veterans 45 years of age and older who have experienced multiple concussions from explosions – sleep disturbance, impairment of the brain’s own natural ability to clear waste products and toxic substances, and the brain adrenaline system. We are studying how these processes may combine to increase the risk of Alzheimer’s disease and other disorders that cause dementia. If we are successful, we will gain information that may help us prevent these devastating diseases.
GLIMPSE OSA: Defining the effect of CPAP therapy for obstructive sleep apnea (OSA) on glymphatic function
Led by Dr. Yeilim Cho, this is a clinical observation study that will evaluate whether standard-of-care treatment for obstructive sleep apnea (OSA) with continuous positive airway pressure (CPAP) therapy improves measures of perivascular fluid exchange, or glymphatic clearance, using non-invasive MRI-based assessment. The proposed study is the first of its kind to 1) define whether individuals with clinical OSA exhibit impaired glymphatic function, 2) whether treatment of OSA by CPAP can improve those measures of glymphatic function, and to seek to define the interrelationships between the effect of CPAP on sleep behavior and glymphatic function. Given the proposed role that impairment of sleep-active glymphatic function plays in the development of Alzheimer's disease-related disorders, this study seeks to find a novel, and potentially important new understanding in the link between sleep disruption, and the development of Alzheimer's pathology.
GLIMPSE Tai Chi: Defining whether Tai Chi practice improves sleep and glymphatic function in the aging human brain
In a collaborative study with Dr. Gail Li, this study will test whether training in Tai Chi, an ancient Chinese martial art characterized by intentional body movement, breathing control, and inward calm, promotes sleep and the clearing of wastes from the brain. These studies may shed light on activities that the middle-aged and elderly may engage in to help prevent or offset the risk of dementing disorders such as Alzheimer’s disease.
Augmented Neurophysiology of Sleep and Performance Readiness
Poor quality and disrupted sleep are common in the aging population, but are also features of many neurological and psychiatric conditions including Alzheimer’s disease and other dementing disorders, concussions, depression, headaches and others. In these conditions, loss of sleep may be both a symptom and a driver of the underlying problem. For example, impairment of the brain waste clearance that occurs during sleep may contribute to the persistence of post-concussive symptoms and the development of the pathology underlying Alzheimer’s disease. In this Department of Defense-sponsored project, we are working with parters at the University of Washington, Brain Electrophysiology Labs, the University of North Carolina, Oregon Health & Science University and Montana State University to test device that uses electrical brain stimulation to improve sleep. Through this work, we will determine whether this stimulation approach improves human brain waste clearance during sleep and can offset the cognitive impacts of sleep disruption.
Development and validation of non-invasive MRI-based measures of glymphatic function
Since its discovery in 2012, the glymphatic system has gained much interest as a potential mechanistic link between sleep disruption and the development of several neurological and psychiatric conditions. This includes the potential role that sleep disruption and slowed glymphatic clearance may play in the development of dementing disorders such as Alzheimer’s disease. Glymphatic function is typically assessed in humans using a difficult and invasive MRI approach requiring injection of contrast agent into the fluid space surrounding the brain, a limitation which is a major barrier to our understanding of the role that glymphatic impairment plays in the development of human disease. In work funded in part through the VA Puget Sound R&D Pilot Program and the Garvey Institute for Brain Health Solutions, collaborative projects with partners at the University of Washington, the VA Puget Sound and Oregon Health & Science University, our group is conducting both preclinical (animal) and clinical (human) imaging studies to develop and validate non-invasive MRI-based measures of glymphatic function.
Changes in Choroid Plexus Function in the Development of Neurodegenerative Disease
Cerebrospinal fluid (CSF) is produced by the choroid plexus, located in the ventricles of the brain, and plays an important role in brain physiology. While the choroid plexus and CSF production can be dynamically regulated, the relationship between sleep states and CSF composition is not well understood. Since both inefficient waste clearance and sleep disruption are prominent features of Alzheimer’s disease this project will determine the role of the choroid plexus in the regulation of sleep-wake glymphatic homeostasis through modulation of CSF dynamics. Combining omics technologies, dynamic in vivo microscopy approaches in animal models, and validation in human choroid plexus this study, funded in part by a Development Project Grant from the UW Alzheimer’s Disease Research Center, will inform prospective therapeutic strategies that will be widely applicable to diseases of impaired protein clearance, including Alzheimer’s disease.
Leducq Network: A Translational Approach Towards Understanding Brain Waste Clearance in Cerebral Amyloid Angiopathy
Cerebral amyloid angiopathy (CAA) is a disease characterized by amyloid deposition in the meningeal and parenchymal blood vessels, coupled with vascular dysfunction, white matter hyperintensities, and ultimately resulting in cerebral hemorrhagic injury. Impairment in CSF clearance has been proposed as a contributor to disease progression, although the mechanism for this is unclear. This project, funded by the Leducq foundation, and in collaboration with the UW Neuropathology Core and neuropathologist Dr Caitlyn Latimer, seeks to characterize the vascular-glymphatic association with pathological features of CAA in human brain tissue. By thorough quantification of the cellular components necessary for glymphatic clearance, and vascular function we aim to better understand the role of CSF dynamics in the setting of CAA.
FORTIFY - From Molecular Physiology to Biophysics of the Glymphatic System: a Regulatory Role for Aquaporin-4 .
Glymphatic function is dependent on the perivascular localization of AQP4 on astrocyte endfeet, and glymphatic impairment has been linked to a number of neurodegenerative conditions. Therefore, pharmacologically targeting AQP4 localization to enhance glymphatic function is of particular interest. However, AQP4 dynamics remain completely understood, and the specificity of current pharmacological approaches is uncertain. This project, in collaboration with Professor Roslyn Bill at Aston University, and funding from …. Aims to address the mechanisms that govern AQP4 localization and thus water and CSF transport in the context of glymphatic function, and diseases associated with glymphatic impairment.